veriseq nipt v2
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. The test offers an option to request the reporting of sex chromosome aneuploidy SCA.

Illumina Introduces Expanded Version Of Veriseq Nipt Solution Offering More Comprehensive Detection Of Rare Chromosomal Conditions Business Wire
The BaseSpace RNA-Seq Alignment App analyzes data from the TruSight RNA Pan-Cancer Panel providing a simple results summary that includes a fusion table variant table and gene expression table.
. Run the RNA-Seq workflow FASTQ only on the MiSeq and stream the data to BaseSpace. Options with VeriSeq NIPT Solution v21. ProductsLearnCompanySupportRecommended Links Products Software Analysis Services Popular Products Instruments Selection Tools.
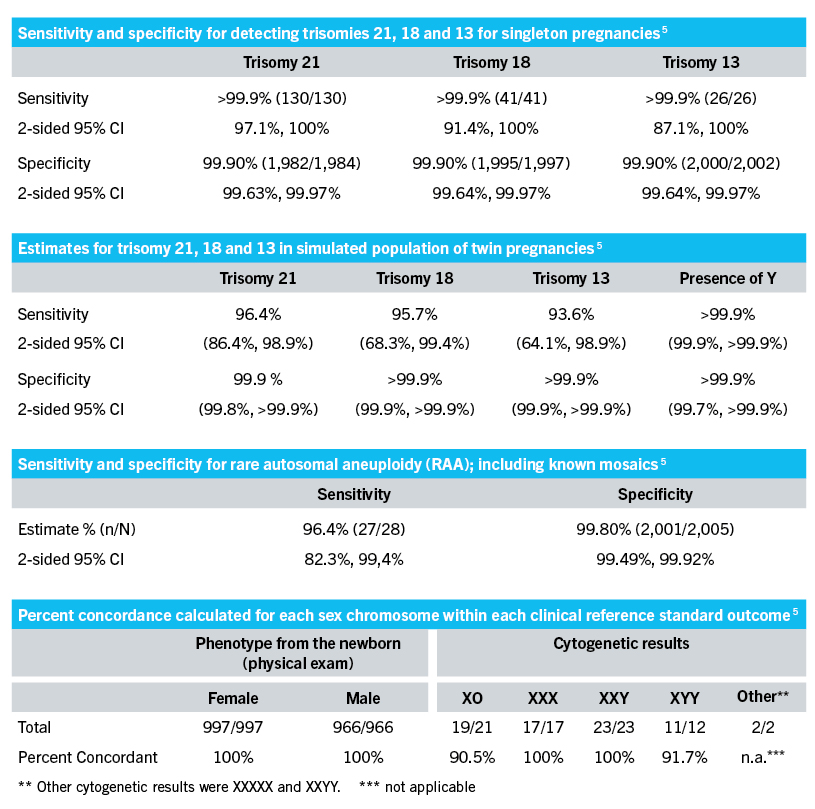
The automated workflow easily scales to analyze 24 48 or 96 samples per run to allow for efficiency and flexibility in managing sample volumes. This CE-IVD software enables clinical labs in the EU to easily analyze sequencing data for noninvasive prenatal testing NIPT in their own lab. The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for.
TableofContents RevisionHistory iii Chapter1VeriSeqNIPTSolutionv2 1 Introduction 1 SystemArchitecture 2 Chapter2VeriSeqNIPTWorkflowManager 4 Introduction 4. VeriSeq NIPT Solution Comprehensive and reliable NIPT solution Reagents instruments and CE-IVD marked library prep and analysisreporting software in an automated workflow for in-lab prenatal aneuploidy screening. The integrated VeriSeq NIPT Solution v2 provides every - thing needed to run the assay.
VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. This product must not be used as the sole basis for diagnosis or other pregnancy management decisions. The VeriSeq NIPT Solution v2 assay enables accurate identification of fetal aneuploidy allowing detection of genome-wide fetal chromosomal anomalies with high clinical sensitivities and specificities and a low assay failure rateClinical Trial Notification CTN.
Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese. Easy-to-use validated CE-IVD marked NIPT analysis software removes the burden of bioinformatics development.
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. The assay provides information about fetal chromosomal status as early as 10.
Instructions for analyzing assay data using the VeriSeq NIPT Solution v2 software. VeriSeq NIPT Solution v2 Consumables Equipment List Consumables and equipment list required for the VeriSeq NIPT Solution v2. Business Wire Illumina has collaborated with Next Generation Genomic NGG Thailand to introduce an automated in-lab IVD solution called VeriSeq NIPT Solution v2 in Thailand.
Equipment Height Width Depth Weight VeriSeqOnsiteServerv2 438 cm 173 in 178 cm 7in 635 cm 25 in 259kg 57lbs VeriSeqNIPT MicrolabSTARwithAutoload 903 cm 356 in 199 cm 783 in 1006 cm 396 in 160kg 353lbs VeriSeqOnsiteServerv2PlacementRequirements PositiontheVeriSeqOnsiteServerv2toallowfor. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese. U VeriSeqNIPTSamplePrepKit24samplespart20025895 u.
Intuitive Illumina Software Illuminas VeriSeq NIPT Workflow Manager Software includes a graphical interface to guide users through protocol selection and assay setup. Týdnu těhotenství nebo později. VeriSeq NIPT Solution v2 is a next-generation sequencing based method to noninvasive prenatal testing Illuminas VeriSeq NIPT Solution v2.
You can also use your own pipeline for analysis. View Options IVD Symbol Key Symbol key and translations for Illumina IVD products. View Options VeriSeq NIPT Solution v2 Software Guide Instructions for use of the software involved with the VeriSeq NIPT Solution v2.
The laboratory can choose to run basic or ge- nome-wide screening by sample. VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. VeriSeq NIPT v2 - Illumina VeriSeq NIPT Solution v2 Aneuploidii plodu pro chromozomy 21 18 13 X a Y lze detekovat s vysokým stupněm přesnosti neinvazivním prenatálním testováním NIPT které využívá celogenomové sekvenování mimo buněčné DNA cfDNA získané z krevní plazmy matky v 10.
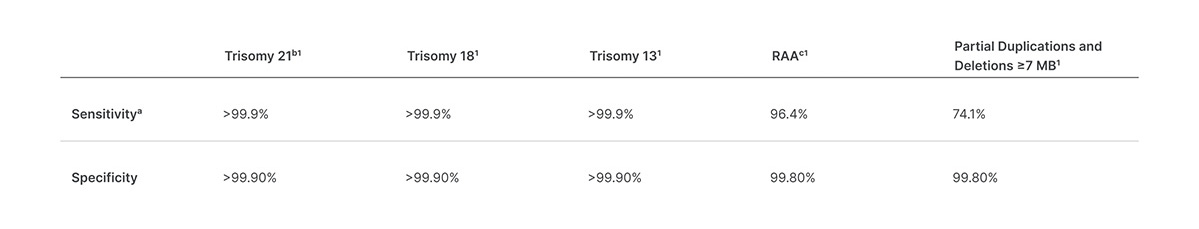
View Options IVD Symbol Key Symbol key and translations for Illumina IVD products. VeriSeq NIPT Analysis Software 16 Samples provides clinical labs in the European Union EU with a fast proven CE-IVD marked solution for analyzing sequencing data for use in noninvasive prenatal testing NIPT. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and deletions for all autosomes and aneuploidy status for all chromosomes.
PDF 1 MB Aug 13 2021. VeriSeq NIPT Solution v2 Consumables Equipment List Consumables and equipment list required for the VeriSeq NIPT Solution v2. This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome.
PDF 1 MB Aug 13 2021. VeriSeq NIPT Solution v2 scales according to your labs needs through customized menu selection for each individual sample and versatile batch options1 With a long-lasting partnership committed to your labs growth and continued success together we can shape the future of prenatal testing. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and deletions for all autosomes and aneuploidy status for all chromosomes.
The test offers an option to request the reporting of sex chromosome aneuploidy SCA. This product must not be used as the sole basis for diagnosis or other pregnancy management decisions. PDF 1 MB Aug 16 2021.
Each run including complete sample tracking is summarized in a downloadable report file. View Options VeriSeq NIPT Solution v2 Software Guide Instructions for use of the software involved with the VeriSeq NIPT Solution v2.

Illumina And Next Generation Genomic Partner To Launch Veriseq Nipt Solution In Thailand

Veriseq Nipt Solution V2 Support

Study Design Dansr Products Produced From Cfdna Were Divided Between Download Scientific Diagram

Noninvasive Prenatal Testing How Far Can We Reach Detecting Fetal Copy Number Variations European Journal Of Obstetrics And Gynecology And Reproductive Biology
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Microlab Star Automated Liquid Handling Hamilton Company
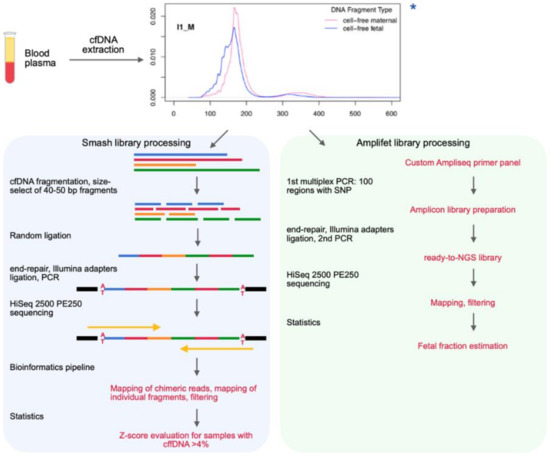
Genes Free Full Text Nipt Technique Based On The Use Of Long Chimeric Dna Reads Html

Distribution Of Fetal Fraction For Samples That Underwent Genome Wide Download Scientific Diagram

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Analysis Of Cfdna Using A Modified Illumina Veriseq Non Invasive Download Scientific Diagram
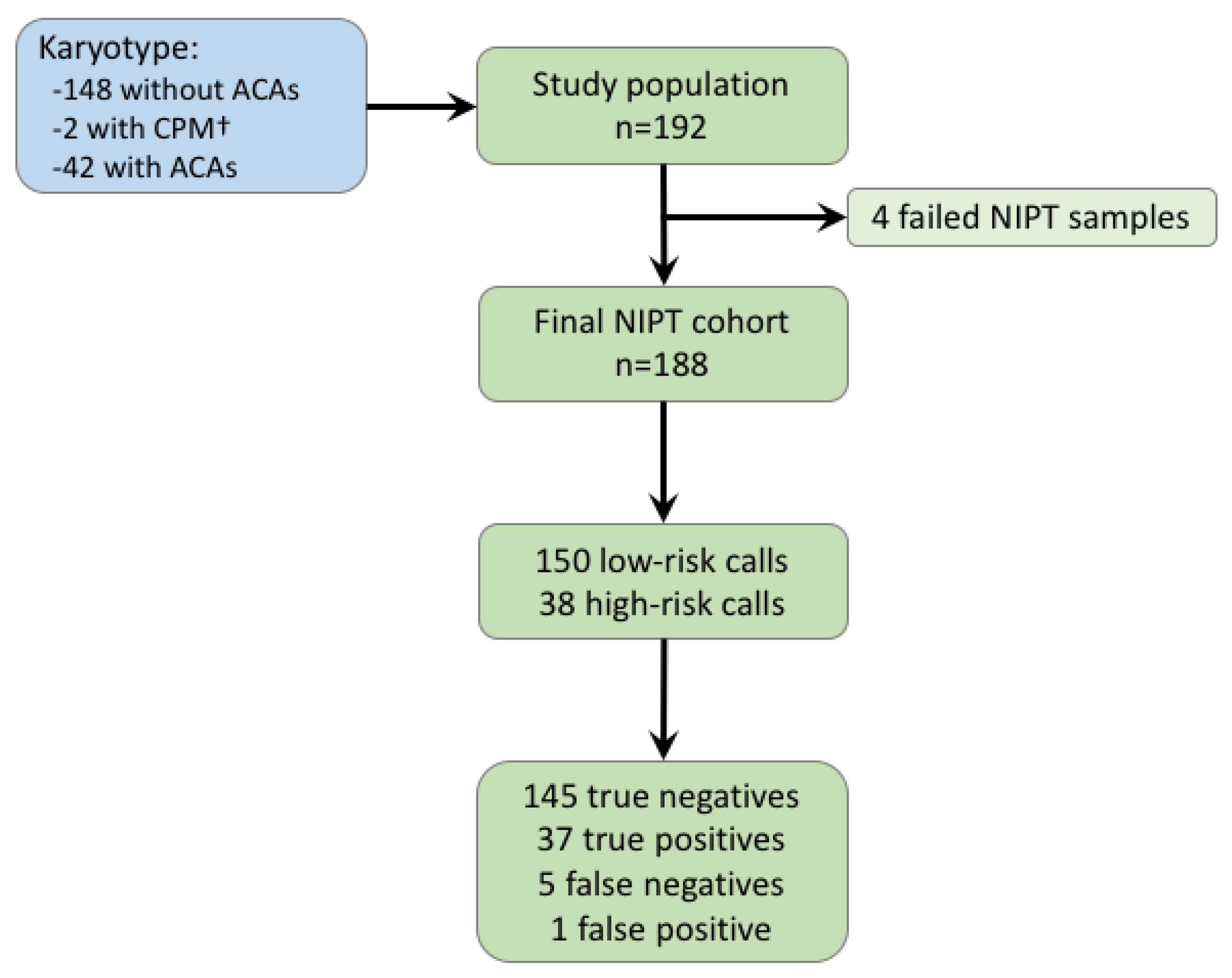
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html

Performance Qualification Praenatest
5524澳门24小时线路 5524澳门24小时线路网站 主頁 欢迎您

Illumina S Noninvasive Prenatal Screening Kit Receives Regulatory Approval In S Korea Business Wire

Illumina On Twitter Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19

Comments
Post a Comment